Are Fatty Acids Formed by Dehydration Reactions
Fatty nitriles have lately become of interest in the framework of biofuels and for the valorization of the oil part of biomass to form fine chemicals or polymers. Formation of disaccharides from monosaccharides in carbohydrates the formation of lipids with one glycerol and three molecules of fatty acids are examples of dehydration synthesis.

Glycosidic Bond Can Form Between Glucose Molecules In A Starch Molecule Through A Dehydration Reaction
The bonds formed during the reaction are single bonds between the fatty.

. Formation of disaccharides from monosaccharides in carbohydrates the formation of lipids with one glycerol and three molecules of fatty acids are examples of dehydration synthesis. Waxes are formed from dehydration reactions of a fatty acid and a long-chain alcohol. Polymerization reactions are good examples of dehydration synthesis reaction in which monomer units condense together to form polymers.
Glycogen Starch and Cellulose. Three molecules of water are released in the process. A fat is formed when three fatty acids join a glycerol by a digestive reaction.
WHAT IS DEHYDRATION SYNTHESIS. As a result of which three water molecules are released and glycerol molecule become. The molecule is formed as water molecule is lost at each point of attachment of.
Dehydration synthesis is a form or chemical reaction between two smaller molecules to form a much larger molecule accompanied by the release of water H2O. The hydroxyl groupsOH of glycerol molecule and carboxyl groupsCOOH of three fatty acids interact with each other. Now these are long chain fatty acids and long chain alcohols on the by the reaction of these two.
Fatty acids are molecules not formed by dehydration reactions. 3 Which of these molecules is not formed by dehydration reactions. A fatty acids B disaccharides C DNA D protein E amylose 4 In animal metabolism most of the monomers released by digestion of food macromolecules are metabolized to provide energy.
Fats and oils are esters of fatty acids and glycerol whereas waxes are esters of fatty acids and long-chain alcohols. Triacylglycerol is formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction. The synthesis of fatty acids takes place in the cytosol.
Triglycerides are made up of one glycerol and three fatty acids. The synthesis of triglycerides takes place within the endoplasmic reticulum ER. The 3 Examples of these are.
A lipid is formed when 3 fatty acid molecules are joined to a glycerol molecule during a dehydration synthesis reaction. To modify the structures you may need to delete atoms or bonds and draw new bonds. 3 This is a dehydration synthesis reaction.
The production of long-chain fatty nitriles by the direct reaction of acids with NH3 has not been extensively studied although several c. Triacylglycerol is formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction. Dehydration reaction Any synthesis reaction which results in the formation of complex molecules from simple molecules is called as dehydrat View the full answer Transcribed image text.
Only a small portion of these monomers are used for synthesis of new macromolecules. This reaction is known as dehydration synthesis. Which of these molecules is not formed by dehydration reactions.
This ones as per the question who access are formed by the reaction of patisserie and alcohol. Foods high in fat are at the top of the food pyramid and these foods have about twice the. Since fats consist of three fatty acids and a glycerol they are also called triacylglycerols or triglycerides.
Triacylglycerol is formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction. Fatty acids In animal metabolism most of the monomers released by digestion of food macromolecules are metabolized to provide energy. Where does triglyceride synthesis occur.
The synthesis of phospholipids occurs on the. Similarly the formation of nucleic acid from nucleotide is also an example of dehydration synthesis. Three molecules of water are released in the process.
So this is the fatty acid containing the group cell function. A triglyceride is also called a simple lipid and is the chemical form in which most fat exists in food as well as in the body. As a result of which three water molecules are released and glycerol molecule become covalently linked with three fatty acids via 3 ester bonds forming triglyceridelipid.
Lipids are molecules that contain a chain of fatty acid a phosphate group and ester linkages as shown. Further detail about this can be seen here. 2 The monomers are fatty acids.
Dehydration synthesis is the process by which polymeric molecules such as proteins. The removal of three water molecules in the process of forming a triglyceride further increases the energy density of the molecule. The esters of fatty acids are formed after the dehydration reaction between the fatty acids and the alcohol molecules.
A the reaction of a fat forming glycerol and fatty acids with the consumption of water B the reaction of two monosaccharides forming a disaccharide with the release of water C the synthesis of a nucleotide from a phosphate a pentose sugar and a nitrogenous base with the production of a molecule of water. Each of the three fatty acids undergoes a dehydration reaction with the alcohol moieties on glycerol to generate one molecule of triglyceride. Triacylglycerol is formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction.
The monomers that are involved in building a long chain of lipids are fatty acids. And this is the alcohol containing the wave function. We are getting waxes so we have to draw the structure of the works formally here.
Draw the structure of the wax formed by modifying the structures shown. Select Draw Rines Erase Fatty acids are carboxylic acids with long hydrophobic tails. Since fats consist of three fatty acids and a glycerol they are also called triacylglycerols or triglycerides.
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition.
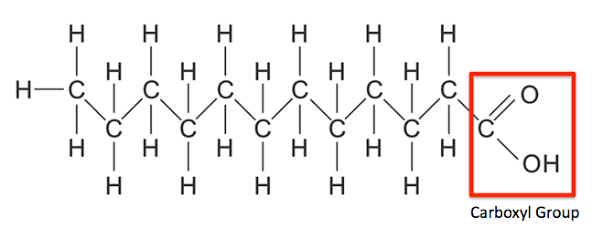
How Does Dehydration Synthesis Relate To Lipids Socratic

Dehydration Reaction To Form A Peptide Bond One Molecule Of Water Is Released Biochemistry Peptide Bond Mcat Prep
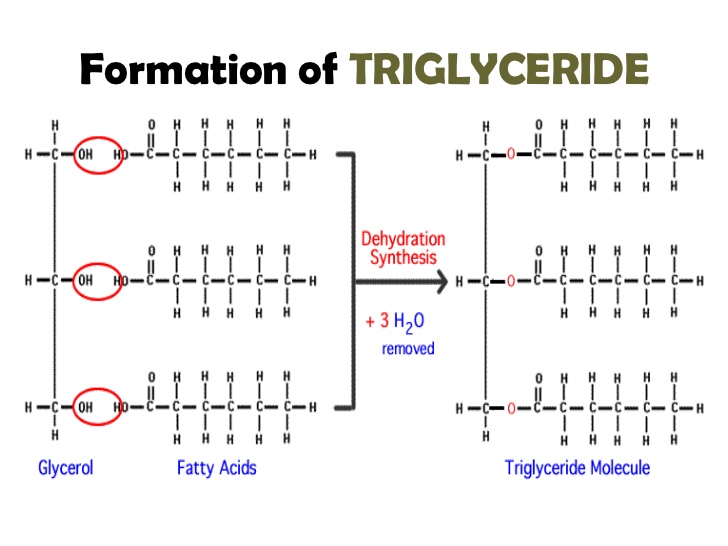
No comments for "Are Fatty Acids Formed by Dehydration Reactions"
Post a Comment